UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 7.01. | Regulation FD Disclosure. |
Galera Therapeutics, Inc. (the “Company”) from time to time presents and/or distributes to the investment community at various industry and other conferences slide presentations to provide updates and summaries of its business. On April 28, 2021, the Company posted an updated corporate slide presentation in the “Investors” portion of its website at www.galeratx.com. A copy of its current corporate slide presentation is attached to this Current Report on Form 8-K as Exhibit 99.1. The Company undertakes no obligation to update, supplement or amend the materials attached hereto as Exhibit 99.1.
The information contained in Item 7.01 of this Form 8-K (including Exhibit 99.1 attached hereto) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly provided by specific reference in such a filing.
| Item 9.01. | Financial Statements and Exhibits. |
(d) Exhibits
The following exhibit relating to Item 7.01 shall be deemed to be furnished, and not filed:
| Exhibit No. |
Description | |
| 99.1 | Corporate Slide Presentation of Galera Therapeutics, Inc. dated April 28, 2021 | |
| 104 | Cover Page Interactive Data File (embedded within the inline XBRL document) | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| GALERA THERAPEUTICS, INC. | ||||||
| Date: April 28, 2021 | By: | /s/ J. Mel Sorensen, M.D. | ||||
| J. Mel Sorensen, M.D. | ||||||
| President and Chief Executive Officer | ||||||

Exhibit 99.1 Transforming radiotherapy for patients with cancer April 2021Exhibit 99.1 Transforming radiotherapy for patients with cancer April 2021

Forward-Looking Statements Certain information contained in this presentation and statements made orally during this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Galera’s own internal estimates and research. While Galera believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Galera believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, the safety, efficacy, regulatory and clinical progress, and therapeutic potential of current and prospective product candidates, plans and timing for the commencement of and the release of data from clinical trials, our plans to prepare for commercialization and a US launch, the anticipated direct and indirect impact of COVID-19 on Galera's business and operations, planned clinical trials and preclinical activities, potential product approvals and related commercial opportunity, current and prospective collaborations, and timing and likelihood of success, plans and objectives of management for future operations, are forward-looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The information in this presentation, including without limitation the forward-looking statements contained herein, represent our views as of the date of this presentation. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. The forward-looking statements in this presentation involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the drug development process and the regulatory approval process, our reliance on third parties over which we may not always have full control, and other important risks and uncertainties that are described in Galera’s Annual Report on Form 10-K for the year ended December 31, 2020 filed with the U.S. Securities Exchange Commission (SEC) and Galera’s other filings with the SEC. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. Whenever the Company uses the terms “transform radiotherapy” or “transforming radiotherapy” in this presentation, it is referring to its mission statement. 2Forward-Looking Statements Certain information contained in this presentation and statements made orally during this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and Galera’s own internal estimates and research. While Galera believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. While Galera believes its internal research is reliable, such research has not been verified by any independent source. This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, the safety, efficacy, regulatory and clinical progress, and therapeutic potential of current and prospective product candidates, plans and timing for the commencement of and the release of data from clinical trials, our plans to prepare for commercialization and a US launch, the anticipated direct and indirect impact of COVID-19 on Galera's business and operations, planned clinical trials and preclinical activities, potential product approvals and related commercial opportunity, current and prospective collaborations, and timing and likelihood of success, plans and objectives of management for future operations, are forward-looking statements. The words ”may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The information in this presentation, including without limitation the forward-looking statements contained herein, represent our views as of the date of this presentation. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. The forward-looking statements in this presentation involve risks and uncertainties that could cause actual results to differ materially from those reflected in such statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the drug development process and the regulatory approval process, our reliance on third parties over which we may not always have full control, and other important risks and uncertainties that are described in Galera’s Annual Report on Form 10-K for the year ended December 31, 2020 filed with the U.S. Securities Exchange Commission (SEC) and Galera’s other filings with the SEC. New risk factors and uncertainties may emerge from time to time, and it is not possible to predict all risk factors and uncertainties. Whenever the Company uses the terms “transform radiotherapy” or “transforming radiotherapy” in this presentation, it is referring to its mission statement. 2

Toxicity Transforming radiotherapy by reducing side effects and increasing anti-cancer efficacy Over 50% of Cancer Patients Receive Radiotherapy 3 EfficacyToxicity Transforming radiotherapy by reducing side effects and increasing anti-cancer efficacy Over 50% of Cancer Patients Receive Radiotherapy 3 Efficacy

Toxicity REDUCING TOXICITY In radiotherapy Galera shifts the balance from normal tissue-damaging high levels of superoxide…. 4Toxicity REDUCING TOXICITY In radiotherapy Galera shifts the balance from normal tissue-damaging high levels of superoxide…. 4

… W HI L E I NCRE A S I NG E F F I CA CY to potentially tumor-toxic high levels of hydrogen peroxide. 5 Efficacy… W HI L E I NCRE A S I NG E F F I CA CY to potentially tumor-toxic high levels of hydrogen peroxide. 5 Efficacy

Transforming Radiotherapy Reducing Increasing Large Market IMRT Toxicity SBRT Efficacy Opportunities In Phase 3 with Breakthrough Encouraging Survival Data High Unmet Medical Need & Therapy Designation in Pancreatic Cancer Trial Limited Therapeutic Options Severe Oral Mucositis Pancreatic Cancer Radiotherapy needed by over In Head & Neck Cancer Locally Advanced half of patients with cancer Esophagitis Lung Cancer Galera building US commercial in Lung Cancer Locally Advanced team for Avasopasem Launch 6Transforming Radiotherapy Reducing Increasing Large Market IMRT Toxicity SBRT Efficacy Opportunities In Phase 3 with Breakthrough Encouraging Survival Data High Unmet Medical Need & Therapy Designation in Pancreatic Cancer Trial Limited Therapeutic Options Severe Oral Mucositis Pancreatic Cancer Radiotherapy needed by over In Head & Neck Cancer Locally Advanced half of patients with cancer Esophagitis Lung Cancer Galera building US commercial in Lung Cancer Locally Advanced team for Avasopasem Launch 6

Robust Pipeline Phase 1 Phase 2 Phase 3 Next Anticipated Milestone Head & Neck ROMAN: Avasopasem vs. Placebo Topline Data: 2H 2021 IMRT induced Cancer Severe Oral 1 Mucositis EUSOM: Avasopasem Topline Data: 2H 2021 IMRT induced Lung AESOP: Avasopasem Topline Data: 1H 2022 2 Esophagitis Cancer SBRT GRECO-1: GC4711 vs. Placebo Initial Data: 1H 2022 3 Combo Pancreatic Pilot: GC4419 vs. Placebo Final Data: 2H 2021 SBRT Cancer 4 Combo GRECO-2: GC4711 vs. Placebo Initiate Trial: 1H 2021 Hospitalized Pilot: GC4419 vs. Placebo Topline Data: 1H 2021 COVID-19 Patients (1) EUSOM is a single-arm multi-center trial evaluating the safety and efficacy of avasopasem in patients with HNC in Europe (2) Phase 2a trial in patients with lung cancer building on avasopasem safety and tolerability findings from SOM trials in patients with HNC (3) Trial to assess anti-cancer efficacy of SBRT +/- GC4711; subsequently, intend to assess anti-cancer efficacy of SBRT and checkpoint inhibitor +/- GC4711 (4) The first SBRT combination trial used GC4419 (avasopasem). Observations from this pilot trial have been used to guide development of GC4711 to assess anti-cancer efficacy in combination with SBRT 7Robust Pipeline Phase 1 Phase 2 Phase 3 Next Anticipated Milestone Head & Neck ROMAN: Avasopasem vs. Placebo Topline Data: 2H 2021 IMRT induced Cancer Severe Oral 1 Mucositis EUSOM: Avasopasem Topline Data: 2H 2021 IMRT induced Lung AESOP: Avasopasem Topline Data: 1H 2022 2 Esophagitis Cancer SBRT GRECO-1: GC4711 vs. Placebo Initial Data: 1H 2022 3 Combo Pancreatic Pilot: GC4419 vs. Placebo Final Data: 2H 2021 SBRT Cancer 4 Combo GRECO-2: GC4711 vs. Placebo Initiate Trial: 1H 2021 Hospitalized Pilot: GC4419 vs. Placebo Topline Data: 1H 2021 COVID-19 Patients (1) EUSOM is a single-arm multi-center trial evaluating the safety and efficacy of avasopasem in patients with HNC in Europe (2) Phase 2a trial in patients with lung cancer building on avasopasem safety and tolerability findings from SOM trials in patients with HNC (3) Trial to assess anti-cancer efficacy of SBRT +/- GC4711; subsequently, intend to assess anti-cancer efficacy of SBRT and checkpoint inhibitor +/- GC4711 (4) The first SBRT combination trial used GC4419 (avasopasem). Observations from this pilot trial have been used to guide development of GC4711 to assess anti-cancer efficacy in combination with SBRT 7

Toxicity Reducing IMRT Toxicity 8Toxicity Reducing IMRT Toxicity 8

Randomized 1:1:1 223 Patient Phase 2b Trial – Robust Results Randomized Placebo-Controlled Severe Oral Mucositis (SOM) Trial Avasopasem 90mg x 7 weeks R Avasopasem 30mg x 7 weeks Placebo x 7 weeks Stratification • Tumor HPV status: + / - • Cisplatin schedule: qwk / q3wk Tumor Outcomes Population Treatment Endpoints • Survival (OS, PFS) • Locoregional control (LRC) • Patients with Head & Neck • Avasopasem 90mg, 30mg, • Primary: Reduction in SOM • Distant Metastases Free (DMF) Cancer (locally advanced) or placebo duration WHO Grading Scale: • Receiving standard IMRT • 60-minute IV infusion just • Secondary: Reduction in and cisplatin over 7 weeks before IMRT SOM incidence & severity 1 2 3 4 No ulcers Ulcers Ulcers Ulcers Erythema & Able to eat Require Unable to soreness solid diet liquid diet eat or drink 9Randomized 1:1:1 223 Patient Phase 2b Trial – Robust Results Randomized Placebo-Controlled Severe Oral Mucositis (SOM) Trial Avasopasem 90mg x 7 weeks R Avasopasem 30mg x 7 weeks Placebo x 7 weeks Stratification • Tumor HPV status: + / - • Cisplatin schedule: qwk / q3wk Tumor Outcomes Population Treatment Endpoints • Survival (OS, PFS) • Locoregional control (LRC) • Patients with Head & Neck • Avasopasem 90mg, 30mg, • Primary: Reduction in SOM • Distant Metastases Free (DMF) Cancer (locally advanced) or placebo duration WHO Grading Scale: • Receiving standard IMRT • 60-minute IV infusion just • Secondary: Reduction in and cisplatin over 7 weeks before IMRT SOM incidence & severity 1 2 3 4 No ulcers Ulcers Ulcers Ulcers Erythema & Able to eat Require Unable to soreness solid diet liquid diet eat or drink 9

Consistent and Encouraging Results Across SOM Endpoints SOM Incidence SOM Duration SOM Severity Reduction in Reduction in Reduction in incidence median days incidence of 34% 47% 92% Grade 4 70% 20 30% 18 60% 25% 16 50% 14 p=0.009* 20% 12 40% p=0.045* 10 15% 30% 8 10% 20% 6 4 10% p=0.024 5% 2 0% 0 0% Placebo 30mg 90mg Placebo 30mg 90mg Placebo 30mg 90mg Intent-To-Treat (ITT) Population *Secondary endpoints (incidence and severity) have nominal p values compared to placebo 10Consistent and Encouraging Results Across SOM Endpoints SOM Incidence SOM Duration SOM Severity Reduction in Reduction in Reduction in incidence median days incidence of 34% 47% 92% Grade 4 70% 20 30% 18 60% 25% 16 50% 14 p=0.009* 20% 12 40% p=0.045* 10 15% 30% 8 10% 20% 6 4 10% p=0.024 5% 2 0% 0 0% Placebo 30mg 90mg Placebo 30mg 90mg Placebo 30mg 90mg Intent-To-Treat (ITT) Population *Secondary endpoints (incidence and severity) have nominal p values compared to placebo 10

Avasopasem Efficacy Significantly Better than Placebo PLACEBO Arm (45 of 74 Pts had ≥1 visit with SOM) 90MG Avasopasem Arm (35 of 76 Pts had ≥1 visit with SOM) RADIOTHERAPY TREATMENT PERIOD FOLLOW UP POST THERAPY RADIOTHERAPY TREATMENT PERIOD FOLLOW UP POST THERAPY 90 mg Avasopasem • 34% Less Incidence SOM • 47% Less Grade 4 OM Grade 3 • 92% Shorter Duration SOM Grade 4 • Delayed Onset of SOM 11Avasopasem Efficacy Significantly Better than Placebo PLACEBO Arm (45 of 74 Pts had ≥1 visit with SOM) 90MG Avasopasem Arm (35 of 76 Pts had ≥1 visit with SOM) RADIOTHERAPY TREATMENT PERIOD FOLLOW UP POST THERAPY RADIOTHERAPY TREATMENT PERIOD FOLLOW UP POST THERAPY 90 mg Avasopasem • 34% Less Incidence SOM • 47% Less Grade 4 OM Grade 3 • 92% Shorter Duration SOM Grade 4 • Delayed Onset of SOM 11

Radiotherapy Efficacy Results Maintained Over Two Years 100% 90% 91% 91% 91% 90% 89% 89% 87% 86% 85% 80% 77% 77% 76% 70% 60% 50% 40% 30% 20% 10% 0% Overall Survival (OS) Progression-Free Survival (PFS) Locoregional Control (LRC) Free of Distant Mets (DMF) PBO 30 mg 90 mg Final ITT Analysis; 2-Year Follow-Up 12Radiotherapy Efficacy Results Maintained Over Two Years 100% 90% 91% 91% 91% 90% 89% 89% 87% 86% 85% 80% 77% 77% 76% 70% 60% 50% 40% 30% 20% 10% 0% Overall Survival (OS) Progression-Free Survival (PFS) Locoregional Control (LRC) Free of Distant Mets (DMF) PBO 30 mg 90 mg Final ITT Analysis; 2-Year Follow-Up 12

Safety Results Comparable to Placebo Avasopasem Generally Well Tolerated Most Frequent AEs Placebo 30 mg 90 mg Avasopasem Avasopasem (any grade) (n=72) (n=73) (n=72) 100 Lymphopenia 89% 92% 88% 99% 97% 97% 90 95% 95% 94% Nausea 75% 68% 82% 80 Fatigue 69% 60% 65% 70 60 Oropharyngeal pain 64% 63% 61% 50 50% Constipation 53% 59% 64% 45% 45% 40 Radiation skin injury 47% 51% 53% 30 20 Vomiting 47% 52% 49% 10 1 1 1 Dysgeusia (taste) 49% 55% 43% 0 Dysphagia 43% 42% 47% Grade 2 or worse Grade 3 or worse Grade 4 or 5 Grade 5 (fatal) Weight decreased 35% 40% 44% Placebo (n=72) 30 mg GC4419 (n=73) 90 mg GC4419 (n=72) Oral candidiasis 29% 45% 43% Leukopenia 39% 37% 39% 13Safety Results Comparable to Placebo Avasopasem Generally Well Tolerated Most Frequent AEs Placebo 30 mg 90 mg Avasopasem Avasopasem (any grade) (n=72) (n=73) (n=72) 100 Lymphopenia 89% 92% 88% 99% 97% 97% 90 95% 95% 94% Nausea 75% 68% 82% 80 Fatigue 69% 60% 65% 70 60 Oropharyngeal pain 64% 63% 61% 50 50% Constipation 53% 59% 64% 45% 45% 40 Radiation skin injury 47% 51% 53% 30 20 Vomiting 47% 52% 49% 10 1 1 1 Dysgeusia (taste) 49% 55% 43% 0 Dysphagia 43% 42% 47% Grade 2 or worse Grade 3 or worse Grade 4 or 5 Grade 5 (fatal) Weight decreased 35% 40% 44% Placebo (n=72) 30 mg GC4419 (n=73) 90 mg GC4419 (n=72) Oral candidiasis 29% 45% 43% Leukopenia 39% 37% 39% 13

Randomized 3:2 450 Patient Phase 3 Trial – Results this Year Avasopasem 90mg x 7 weeks Randomized Placebo-Controlled Severe Oral Mucositis Trial R Placebo x 7 weeks Stratification • Surgery status: + / - before Rx • Cisplatin schedule: qwk / q3wk Tumor Outcomes Population Treatment Endpoints • Survival (OS, PFS) • Locoregional control (LRC) • Patients with Head & Neck • Avasopasem 90mg or • Primary: Reduction in SOM • Distant Metastases Free (DMF) Cancer (locally advanced) placebo incidence WHO Grading Scale: • Receiving standard IMRT • 60-minute IV infusion just • Secondary: Reduction in and cisplatin over 7 weeks before IMRT SOM duration & severity 1 2 3 4 No ulcers Ulcers Ulcers Ulcers Erythema & Able to eat Require Unable to soreness solid diet liquid diet eat or drink 14Randomized 3:2 450 Patient Phase 3 Trial – Results this Year Avasopasem 90mg x 7 weeks Randomized Placebo-Controlled Severe Oral Mucositis Trial R Placebo x 7 weeks Stratification • Surgery status: + / - before Rx • Cisplatin schedule: qwk / q3wk Tumor Outcomes Population Treatment Endpoints • Survival (OS, PFS) • Locoregional control (LRC) • Patients with Head & Neck • Avasopasem 90mg or • Primary: Reduction in SOM • Distant Metastases Free (DMF) Cancer (locally advanced) placebo incidence WHO Grading Scale: • Receiving standard IMRT • 60-minute IV infusion just • Secondary: Reduction in and cisplatin over 7 weeks before IMRT SOM duration & severity 1 2 3 4 No ulcers Ulcers Ulcers Ulcers Erythema & Able to eat Require Unable to soreness solid diet liquid diet eat or drink 14

SOM Market OpportunitySOM Market Opportunity

Head and Neck Cancer – Large Market Opportunity Severe Oral Mucositis is most burdensome side effect – 70% get SOM 650,000 Global Head & Neck Cancer Incidence Initial 65,630 Target US Patients Diagnosed each year Population 42,000 US Patients at Risk for RT-related SOM Locally advanced HNC curable with the standard-of-care IMRT and cisplatin regimen 16Head and Neck Cancer – Large Market Opportunity Severe Oral Mucositis is most burdensome side effect – 70% get SOM 650,000 Global Head & Neck Cancer Incidence Initial 65,630 Target US Patients Diagnosed each year Population 42,000 US Patients at Risk for RT-related SOM Locally advanced HNC curable with the standard-of-care IMRT and cisplatin regimen 16

Head and Neck Cancer Can Affect Anyone Babe Ruth, Lana Turner, Jamie Dimon, Ulysses S. Grant, Sigmund Freud, Humphrey Bogart, Grover Cleveland, Eddie Van Halen Sammy Davis Jr., George Harrison, Michael Douglas, Ann Richards, Tony Gwynn 17Head and Neck Cancer Can Affect Anyone Babe Ruth, Lana Turner, Jamie Dimon, Ulysses S. Grant, Sigmund Freud, Humphrey Bogart, Grover Cleveland, Eddie Van Halen Sammy Davis Jr., George Harrison, Michael Douglas, Ann Richards, Tony Gwynn 17
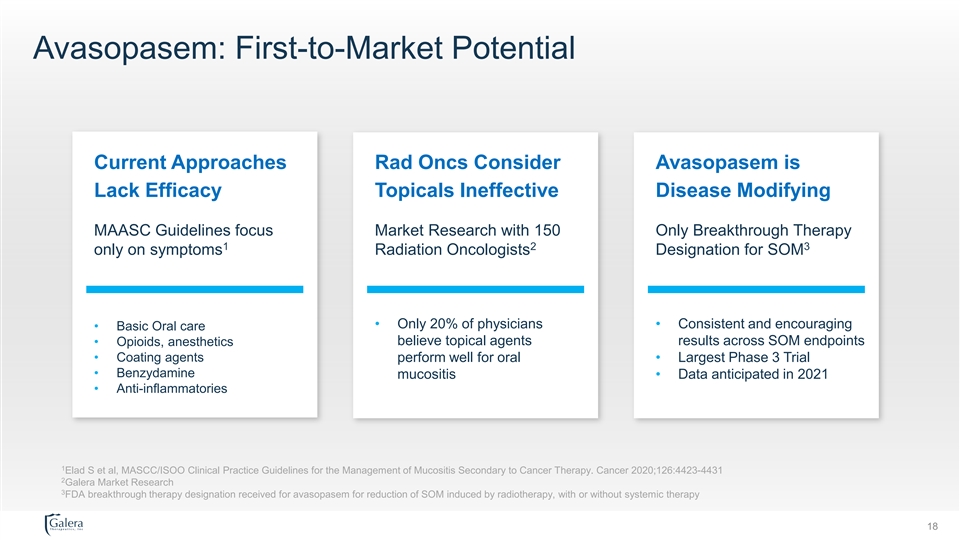
Avasopasem: First-to-Market Potential Current Approaches Rad Oncs Consider Avasopasem is Lack Efficacy Topicals Ineffective Disease Modifying MAASC Guidelines focus Market Research with 150 Only Breakthrough Therapy 1 2 3 only on symptoms Radiation Oncologists Designation for SOM • Only 20% of physicians • Consistent and encouraging • Basic Oral care believe topical agents results across SOM endpoints • Opioids, anesthetics • Coating agents perform well for oral • Largest Phase 3 Trial • Benzydamine mucositis • Data anticipated in 2021 • Anti-inflammatories 1 Elad S et al, MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020;126:4423-4431 2 Galera Market Research 3 FDA breakthrough therapy designation received for avasopasem for reduction of SOM induced by radiotherapy, with or without systemic therapy 18Avasopasem: First-to-Market Potential Current Approaches Rad Oncs Consider Avasopasem is Lack Efficacy Topicals Ineffective Disease Modifying MAASC Guidelines focus Market Research with 150 Only Breakthrough Therapy 1 2 3 only on symptoms Radiation Oncologists Designation for SOM • Only 20% of physicians • Consistent and encouraging • Basic Oral care believe topical agents results across SOM endpoints • Opioids, anesthetics • Coating agents perform well for oral • Largest Phase 3 Trial • Benzydamine mucositis • Data anticipated in 2021 • Anti-inflammatories 1 Elad S et al, MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 2020;126:4423-4431 2 Galera Market Research 3 FDA breakthrough therapy designation received for avasopasem for reduction of SOM induced by radiotherapy, with or without systemic therapy 18

Concentrated Physician Population SOM is Most Burdensome Side Effect of Curative IMRT + Cisplatin Regimen 5,000 72% Radiation Oncologists Sites with Existing 1 in U.S Infusion Capability Initial 2,500 64% Sales Radiotherapy Focus Market Patient Share Treatment Sites 700 Top centers where >80% HNC Patients are treated 1 38% IMRT centers currently infuse drugs 34% more coordinate with medical oncology to infuse patients Additional 17% can add capabilities to infuse patients 1 Primary market research with 125 IMRT centers in the US 19Concentrated Physician Population SOM is Most Burdensome Side Effect of Curative IMRT + Cisplatin Regimen 5,000 72% Radiation Oncologists Sites with Existing 1 in U.S Infusion Capability Initial 2,500 64% Sales Radiotherapy Focus Market Patient Share Treatment Sites 700 Top centers where >80% HNC Patients are treated 1 38% IMRT centers currently infuse drugs 34% more coordinate with medical oncology to infuse patients Additional 17% can add capabilities to infuse patients 1 Primary market research with 125 IMRT centers in the US 19

Where Patients with Head & Neck Cancer are Treated 76% Treated in only 29% Zip Code Areas Galera Market Research (122 Zip Codes are 0) 20Where Patients with Head & Neck Cancer are Treated 76% Treated in only 29% Zip Code Areas Galera Market Research (122 Zip Codes are 0) 20

Most IMRT Centers Have Ability to Infuse Today 72% Radiotherapy Sites Have Existing Infusion Capability Adoption Archetype Determinants A B C D Rad Oncs Have Med Oncs Administer Rad Oncs Need to Rad Oncs Unlikely to Current Capabilities Infusions for Rad Onc Add Capabilities Add Capabilities Avasopasem Infusion Owner Rad Onc Med Onc Rad Onc - MD-Stated Patient Volume High Low High Moderate Ease of Coordination Today High High Low Low Likelihood of Prescribing Avasopasem High High High Low Total % Sample Distribution (n) 38% 34% 17% 11% (51) (39) (23) (12) Data in above table based on primary market research with 125 IMRT centers in the US 21Most IMRT Centers Have Ability to Infuse Today 72% Radiotherapy Sites Have Existing Infusion Capability Adoption Archetype Determinants A B C D Rad Oncs Have Med Oncs Administer Rad Oncs Need to Rad Oncs Unlikely to Current Capabilities Infusions for Rad Onc Add Capabilities Add Capabilities Avasopasem Infusion Owner Rad Onc Med Onc Rad Onc - MD-Stated Patient Volume High Low High Moderate Ease of Coordination Today High High Low Low Likelihood of Prescribing Avasopasem High High High Low Total % Sample Distribution (n) 38% 34% 17% 11% (51) (39) (23) (12) Data in above table based on primary market research with 125 IMRT centers in the US 21

US Radiation Oncologists Trending Away from Private Practice 53% 41% 38% 31% 17% 11% Private Practice Hospital Academic/University 2012 2017 1 Data from ASTRO 22US Radiation Oncologists Trending Away from Private Practice 53% 41% 38% 31% 17% 11% Private Practice Hospital Academic/University 2012 2017 1 Data from ASTRO 22

Favorable Payer Landscape Price strategy intended to $40,000 optimize patient access Additional medical expenses incurred Head and neck cancer not a focus for cost by patients who develop OM control measure $15-25K Step Edits Unlikely Indicative price of full course of therapy High unmet need with limited treatment based on initial payer research options 23Favorable Payer Landscape Price strategy intended to $40,000 optimize patient access Additional medical expenses incurred Head and neck cancer not a focus for cost by patients who develop OM control measure $15-25K Step Edits Unlikely Indicative price of full course of therapy High unmet need with limited treatment based on initial payer research options 23

Esophagitis in Lung Cancer 50% patients get Grade 2 or worse 2,500,000 Global NSCLC Incidence Initial 175,000 Target US Patients Diagnosed each year Population 50,000 US Patients at Risk for RT-related Esophagitis Locally advanced NSCLC frequently treated with IMRT and chemotherapy 24Esophagitis in Lung Cancer 50% patients get Grade 2 or worse 2,500,000 Global NSCLC Incidence Initial 175,000 Target US Patients Diagnosed each year Population 50,000 US Patients at Risk for RT-related Esophagitis Locally advanced NSCLC frequently treated with IMRT and chemotherapy 24

Increasing SBRT Efficacy EfficacyIncreasing SBRT Efficacy Efficacy

People we Have Lost to Pancreatic Cancer Pavarotti, Donna Reed, Dizzy Gillespie, Cardinal Bernardin, Eiko Ishioka, Bonanza’s Pernell Roberts, Joan Crawford Ben Gazzara, Alex Trebek, Alan Bates, Jack Benny, Dr. Sydney Salmon, Billy Paul, Rand Pausch (last lecture) Ruth Bader Ginsburg, John Lewis, Henry Mancini, Sally Ride, Munster’s Fred Gwynne, Columnist William Safire, Michal Landon 26People we Have Lost to Pancreatic Cancer Pavarotti, Donna Reed, Dizzy Gillespie, Cardinal Bernardin, Eiko Ishioka, Bonanza’s Pernell Roberts, Joan Crawford Ben Gazzara, Alex Trebek, Alan Bates, Jack Benny, Dr. Sydney Salmon, Billy Paul, Rand Pausch (last lecture) Ruth Bader Ginsburg, John Lewis, Henry Mancini, Sally Ride, Munster’s Fred Gwynne, Columnist William Safire, Michal Landon 26

Pancreatic Cancer High Unmet Medical Need With Limited Therapeutic Options 500,000 Global Incidence Initial 60,000 Target US Patients Diagnosed each year Population 18,000 Patients with Unresectable Locally Advanced Tumors 5-year survival rate only ~10% SBRT use increasing for locoregional control of pancreatic cancer 27Pancreatic Cancer High Unmet Medical Need With Limited Therapeutic Options 500,000 Global Incidence Initial 60,000 Target US Patients Diagnosed each year Population 18,000 Patients with Unresectable Locally Advanced Tumors 5-year survival rate only ~10% SBRT use increasing for locoregional control of pancreatic cancer 27

Design Pilot Trial in Pancreatic Cancer 42-Patient Double-blind, Placebo-controlled, Randomized Trial SBRT + GC4419 90mg x 5 doses R SBRT+ Placebo x 5 doses 42 Patients Single Multi Center Center Placebo N=8 N=10 GC4419 N=11 N=13 >1 Year >6 Months Population Treatment Endpoints Follow-Up Follow-Up • Patients with Locally-advanced • High-Dose Stereotactic RT • Safety and Feasibility of Enrolling Centers Pancreatic Cancer (LAPC) (SBRT) 10-11Gy x 5 doses dismutase mimetic with SBRT • MD Anderson, Houston, TX • Enrolled after 4-6 months of • 60-minute IV infusion of 90mg • Survival (OS, PFS) • Moffitt Cancer Center, Tampa, FL chemotherapy GC4419 or placebo • Response Rate • UT Southwestern, Dallas, TX • Tumor Control (DMC, LRC) • Duke University, Durham, NC 28Design Pilot Trial in Pancreatic Cancer 42-Patient Double-blind, Placebo-controlled, Randomized Trial SBRT + GC4419 90mg x 5 doses R SBRT+ Placebo x 5 doses 42 Patients Single Multi Center Center Placebo N=8 N=10 GC4419 N=11 N=13 >1 Year >6 Months Population Treatment Endpoints Follow-Up Follow-Up • Patients with Locally-advanced • High-Dose Stereotactic RT • Safety and Feasibility of Enrolling Centers Pancreatic Cancer (LAPC) (SBRT) 10-11Gy x 5 doses dismutase mimetic with SBRT • MD Anderson, Houston, TX • Enrolled after 4-6 months of • 60-minute IV infusion of 90mg • Survival (OS, PFS) • Moffitt Cancer Center, Tampa, FL chemotherapy GC4419 or placebo • Response Rate • UT Southwestern, Dallas, TX • Tumor Control (DMC, LRC) • Duke University, Durham, NC 28

Highlights of Current Analysis Follow-up through at least 6 months on all patients Surgical Resection • 5/24 on GC All with clear tumor margins • 2/18 on PBO 1 with clear tumor margins 85% Increase in 2.5-fold Increase in 2-fold Increase in Overall Survival Response Rate Time to Metastases Hazard Ratios (GC vs. PBO) Survival Response Metastases OS 0.4 PFS 0.4 Median Overall Survival Partial Response Rate Median Time to Mets GC 20.1 Mos GC 29% GC 13.9 Mos LRC 0.3 PBO 10.9 Mos PBO 11% PBO 7.0 Mos DMC 0.3 OS = Overall Survival PFS = Progression-Free Survival LRC = Locoregional Control DMC = Control of Distant Metastases Median follow-up of 9 months as of this data analysis (maximum follow-up 32 months) 29Highlights of Current Analysis Follow-up through at least 6 months on all patients Surgical Resection • 5/24 on GC All with clear tumor margins • 2/18 on PBO 1 with clear tumor margins 85% Increase in 2.5-fold Increase in 2-fold Increase in Overall Survival Response Rate Time to Metastases Hazard Ratios (GC vs. PBO) Survival Response Metastases OS 0.4 PFS 0.4 Median Overall Survival Partial Response Rate Median Time to Mets GC 20.1 Mos GC 29% GC 13.9 Mos LRC 0.3 PBO 10.9 Mos PBO 11% PBO 7.0 Mos DMC 0.3 OS = Overall Survival PFS = Progression-Free Survival LRC = Locoregional Control DMC = Control of Distant Metastases Median follow-up of 9 months as of this data analysis (maximum follow-up 32 months) 29

Median Overall Survival Increased 85% Encouraging hazard ratios for both OS and PFS 1 Overall Survival (OS) Progression-Free Survival (PFS) Median OS GC 20.1 Mos PBO 10.9 Mos Hazard Ratio = 0.4 Hazard Ratio = 0.4 1 PFS defined as local progression or distant metastasis; not censored for treatment post SBRT 30Median Overall Survival Increased 85% Encouraging hazard ratios for both OS and PFS 1 Overall Survival (OS) Progression-Free Survival (PFS) Median OS GC 20.1 Mos PBO 10.9 Mos Hazard Ratio = 0.4 Hazard Ratio = 0.4 1 PFS defined as local progression or distant metastasis; not censored for treatment post SBRT 30

Partial Response Rate Increased 2.5-fold Best Local Response with follow-up through at least 6 months on all patients (ITT, n=42) SBRT + GC SBRT + PBO 40% 30% 20% 10% NE R0 R0 R0 NE R1 0% R0 R0 R0 -10% -20% -30% PR*: 29% (7 of 24 patients) PR*: 11% (2 of 18 patients) -40% -50% -60% R0 R1 = Surgical resection (R0 = clear margins). = Surgical resection (R1 = tumor at margins). *Partial response per modified RECIST NE = not evaluable. (Response Evaluation Criteria in Solid Tumors) 31Partial Response Rate Increased 2.5-fold Best Local Response with follow-up through at least 6 months on all patients (ITT, n=42) SBRT + GC SBRT + PBO 40% 30% 20% 10% NE R0 R0 R0 NE R1 0% R0 R0 R0 -10% -20% -30% PR*: 29% (7 of 24 patients) PR*: 11% (2 of 18 patients) -40% -50% -60% R0 R1 = Surgical resection (R0 = clear margins). = Surgical resection (R1 = tumor at margins). *Partial response per modified RECIST NE = not evaluable. (Response Evaluation Criteria in Solid Tumors) 31
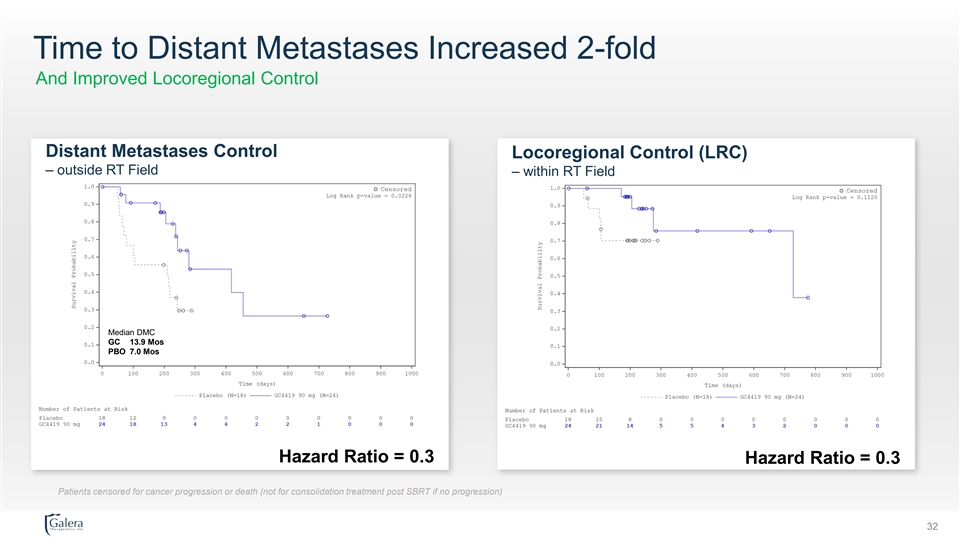
Time to Distant Metastases Increased 2-fold And Improved Locoregional Control Distant Metastases Control Locoregional Control (LRC) – outside RT Field – within RT Field Median DMC GC 13.9 Mos PBO 7.0 Mos Hazard Ratio = 0.3 Hazard Ratio = 0.3 Patients censored for cancer progression or death (not for consolidation treatment post SBRT if no progression) 32Time to Distant Metastases Increased 2-fold And Improved Locoregional Control Distant Metastases Control Locoregional Control (LRC) – outside RT Field – within RT Field Median DMC GC 13.9 Mos PBO 7.0 Mos Hazard Ratio = 0.3 Hazard Ratio = 0.3 Patients censored for cancer progression or death (not for consolidation treatment post SBRT if no progression) 32

Regimen Generally Well Tolerated Toxicity reports through first 90 days after SBRT (ITT, n=42) Placebo Avasopasem Acute Adverse Events (up to 90 days post SBRT) (n=18) (n=24) Grade 3+ AEs 4 (22%) 6 (25%) 1 Grade 3 Gastrointestinal AEs 2 (11%) 2 (8%) 1 No bleeding ulcers by 12-week endoscopy, no GI toxicity > Grade 3 33Regimen Generally Well Tolerated Toxicity reports through first 90 days after SBRT (ITT, n=42) Placebo Avasopasem Acute Adverse Events (up to 90 days post SBRT) (n=18) (n=24) Grade 3+ AEs 4 (22%) 6 (25%) 1 Grade 3 Gastrointestinal AEs 2 (11%) 2 (8%) 1 No bleeding ulcers by 12-week endoscopy, no GI toxicity > Grade 3 33

GRECO-1 Next Steps SBRT + GC4711 100mg x 5 doses R SBRT+ Placebo x 5 doses • Placebo-controlled multicenter trial • Locally Advanced NSC Lung Cancer – Proof o1. f Efficacy results from blinded controlled trial consistent with large & central tumors Concept preclinical studies that showed synergy with RT • 71 Patients • Status: Open & Recruiting Patients Consisten 1.t Magnitude of synergy with RT and consistency across efficacy GRECO-2 Synergy parameters is very encouraging SBRT + GC4711 100mg x 5 doses R SBRT+ Placebo x 5 doses GRECO 1. Galera advanced its dismutase mimetics into larger placebo- • Placebo-controlled multicenter trial Trials controlled trials, in pancreatic and lung cancer • Locally Advanced Pancreatic Cancer – following neoadjuvant chemotherapy • 160 Patients • Status: Soon to open to enrollment 34GRECO-1 Next Steps SBRT + GC4711 100mg x 5 doses R SBRT+ Placebo x 5 doses • Placebo-controlled multicenter trial • Locally Advanced NSC Lung Cancer – Proof o1. f Efficacy results from blinded controlled trial consistent with large & central tumors Concept preclinical studies that showed synergy with RT • 71 Patients • Status: Open & Recruiting Patients Consisten 1.t Magnitude of synergy with RT and consistency across efficacy GRECO-2 Synergy parameters is very encouraging SBRT + GC4711 100mg x 5 doses R SBRT+ Placebo x 5 doses GRECO 1. Galera advanced its dismutase mimetics into larger placebo- • Placebo-controlled multicenter trial Trials controlled trials, in pancreatic and lung cancer • Locally Advanced Pancreatic Cancer – following neoadjuvant chemotherapy • 160 Patients • Status: Soon to open to enrollment 34

SBRT for Non-Small Cell Lung Cancer SBRT is an established treatment for central and large peripheral NSCLC tumors 2,500,000 Global NSCLC Incidence All SBRT 14,600 12,120 15,430 Node- Peripheral Central Central Negative Tumor Tumor Tumor NSCLC >3cm <3cm >3cm Surgery 42,000 175,000 16% 30% 12% ONLY Receive US Patients Diagnosed each year SBRT SBRT (+/- other 81% 67% 85% Today modalities) Other 3% 2% 4% 55,100 Node-Negative NSCLC 35SBRT for Non-Small Cell Lung Cancer SBRT is an established treatment for central and large peripheral NSCLC tumors 2,500,000 Global NSCLC Incidence All SBRT 14,600 12,120 15,430 Node- Peripheral Central Central Negative Tumor Tumor Tumor NSCLC >3cm <3cm >3cm Surgery 42,000 175,000 16% 30% 12% ONLY Receive US Patients Diagnosed each year SBRT SBRT (+/- other 81% 67% 85% Today modalities) Other 3% 2% 4% 55,100 Node-Negative NSCLC 35

Corporate HighlightsCorporate Highlights

Robust Pipeline Phase 1 Phase 2 Phase 3 Next Anticipated Milestone Head & Neck ROMAN: Avasopasem vs. Placebo Topline Data: 2H 2021 IMRT induced Cancer Severe Oral 1 Mucositis EUSOM: Avasopasem Topline Data: 2H 2021 IMRT induced Lung AESOP: Avasopasem Topline Data: 1H 2022 2 Esophagitis Cancer SBRT GRECO-1: GC4711 vs. Placebo Initial Data: 1H 2022 3 Combo Pancreatic Pilot: GC4419 vs. Placebo Final Data: 2H 2021 SBRT Cancer 4 Combo GRECO-2: GC4711 vs. Placebo Initiate Trial: 1H 2021 Hospitalized Pilot: GC4419 vs. Placebo Topline Data: 1H 2021 COVID-19 Patients (1) EUSOM is a single-arm multi-center trial evaluating the safety and efficacy of avasopasem in patients with HNC in Europe (2) Phase 2a trial in patients with lung cancer building on avasopasem safety and tolerability findings from SOM trials in patients with HNC (3) Trial to assess anti-cancer efficacy of SBRT +/- GC4711; subsequently, intend to assess anti-cancer efficacy of SBRT and checkpoint inhibitor +/- GC4711 (4) The first SBRT combination trial used GC4419 (avasopasem). Observations from this pilot trial have been used to guide development of GC4711 to assess anti-cancer efficacy in combination with SBRT 37Robust Pipeline Phase 1 Phase 2 Phase 3 Next Anticipated Milestone Head & Neck ROMAN: Avasopasem vs. Placebo Topline Data: 2H 2021 IMRT induced Cancer Severe Oral 1 Mucositis EUSOM: Avasopasem Topline Data: 2H 2021 IMRT induced Lung AESOP: Avasopasem Topline Data: 1H 2022 2 Esophagitis Cancer SBRT GRECO-1: GC4711 vs. Placebo Initial Data: 1H 2022 3 Combo Pancreatic Pilot: GC4419 vs. Placebo Final Data: 2H 2021 SBRT Cancer 4 Combo GRECO-2: GC4711 vs. Placebo Initiate Trial: 1H 2021 Hospitalized Pilot: GC4419 vs. Placebo Topline Data: 1H 2021 COVID-19 Patients (1) EUSOM is a single-arm multi-center trial evaluating the safety and efficacy of avasopasem in patients with HNC in Europe (2) Phase 2a trial in patients with lung cancer building on avasopasem safety and tolerability findings from SOM trials in patients with HNC (3) Trial to assess anti-cancer efficacy of SBRT +/- GC4711; subsequently, intend to assess anti-cancer efficacy of SBRT and checkpoint inhibitor +/- GC4711 (4) The first SBRT combination trial used GC4419 (avasopasem). Observations from this pilot trial have been used to guide development of GC4711 to assess anti-cancer efficacy in combination with SBRT 37

Back-up Slides Mechanistic and Preclinical DataBack-up Slides Mechanistic and Preclinical Data

Efficacy Differential Effect of Dismutase Mimetics Conversion of superoxide to hydrogen peroxide leverages inherent tissue differences Hydrogen Superoxide Shifting the Peroxide (O ) is more 2 (H O ) is Balance 2 2 damaging more toxic H O 2 2 to normal to cancer cells than cells than cancer normal 1 cells 2 O cells 2 1 Sonis S. Drug Design, Development and Therapy 2021:15 1021–1029 2 Park WH: Oncol Rep 40: 1787-1794, 2018 39 ToxicityEfficacy Differential Effect of Dismutase Mimetics Conversion of superoxide to hydrogen peroxide leverages inherent tissue differences Hydrogen Superoxide Shifting the Peroxide (O ) is more 2 (H O ) is Balance 2 2 damaging more toxic H O 2 2 to normal to cancer cells than cells than cancer normal 1 cells 2 O cells 2 1 Sonis S. Drug Design, Development and Therapy 2021:15 1021–1029 2 Park WH: Oncol Rep 40: 1787-1794, 2018 39 Toxicity

Protection from Lethal Radiation Exposure Observed in Preclinical Studies – Total Body Irradiation (8.5 Gy) to Mice Thompson, et al., Free Radical Research, 44(5):529-540, 2010 40Protection from Lethal Radiation Exposure Observed in Preclinical Studies – Total Body Irradiation (8.5 Gy) to Mice Thompson, et al., Free Radical Research, 44(5):529-540, 2010 40

Synergy with High-Dose RT (SBRT) High-fraction focal irradiation of human tumor xenografts (H1299 NSCLC) in mice RT with Biological Equivalent Doses Vehicle Days Post Treatment Days Post Treatment Days Post Treatment Days Post Treatment SBRT Stereotactic Body Radiation Therapy Sishc BJ et al, Proc. AACR 2020 #6284 41Synergy with High-Dose RT (SBRT) High-fraction focal irradiation of human tumor xenografts (H1299 NSCLC) in mice RT with Biological Equivalent Doses Vehicle Days Post Treatment Days Post Treatment Days Post Treatment Days Post Treatment SBRT Stereotactic Body Radiation Therapy Sishc BJ et al, Proc. AACR 2020 #6284 41

H O build-up in Cancer Cell à Synergy with SBRT 2 2 CAT Synergy abrogated with doxycycline-induced catalase in genetically modified H1299 cells Sishc BJ et al, AACR, 2018 42H O build-up in Cancer Cell à Synergy with SBRT 2 2 CAT Synergy abrogated with doxycycline-induced catalase in genetically modified H1299 cells Sishc BJ et al, AACR, 2018 42

Pancreatic Tumor Model à Synergy with SBRT Marked synergy of Dismutase Mimetic with 12 Gray Radiotherapy PANC-1 PDAC xenograft Sishc BJ et al, AACR Pancreatic Cancer, 2019 43Pancreatic Tumor Model à Synergy with SBRT Marked synergy of Dismutase Mimetic with 12 Gray Radiotherapy PANC-1 PDAC xenograft Sishc BJ et al, AACR Pancreatic Cancer, 2019 43

Enhanced Checkpoint Inhibitor Activity in Vivo GC4419 enhanced tumor response to SBRT + anti-PD-L1, PD-1 or CTLA-4 – within and outside RT field LLC syngeneic lung tumor model Galera Data on file 44Enhanced Checkpoint Inhibitor Activity in Vivo GC4419 enhanced tumor response to SBRT + anti-PD-L1, PD-1 or CTLA-4 – within and outside RT field LLC syngeneic lung tumor model Galera Data on file 44
